Abstract
Introduction: Outcomes in multiple myeloma have significantly improved in recent times owing to the availability of newer more effective therapies. Autologous stem cell transplantation (ASCT) has been used for over three decades to treat multiple myeloma. We aimed to evaluate the outcome of patients receiving ASCT at our institution between 1990 and 2015.
Patients and Methods: A total of 2025 patients received ASCT at Mayo Clinic Rochester between 1st January 1990 and 31st December 2015. The patients were divided in to three cohorts of approximately equal time period, Cohort 1 (1990-1999, n=144), Cohort 2 (2000-2009, n=909) and Cohort 3 (2010-2015, n=972). Outcomes of interest were early mortality, progression free survival (PFS) and overall survival (OS). Disease characteristics were documented from time of diagnosis and PFS and OS were calculated from date of ASCT unless otherwise stated.
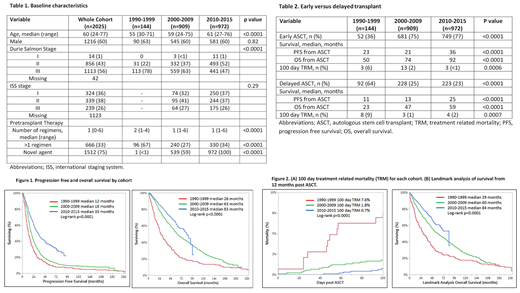
Results: Median age for the whole cohort was 60 years (range 24-77) and increased over time (55 Cohort 1 vs 59 Cohort 2 vs 61 Cohort 3, p<0.0001). The proportion of males (60% for whole cohort) was not significantly different between the 3 time periods. Almost all patients (99%, n=2018) received chemotherapy prior to transplantation (median lines of therapy 1, range 0-6). The number of patients receiving >1 line of therapy prior to transplantation decreased over time (67% Cohort 1 vs 27% Cohort 2 vs 34% Cohort 3, p<0.0001). The use of novel agents was congruous with their availability and increased over time (0% Cohort 1 vs 59% Cohort 2 vs 100% Cohort 3, p<0.000).
After a median follow up of 84 months (95% CI, 78-91 months), median PFS and OS for the whole cohort were 24 and 66 months respectively. Both PFS and OS improved significantly over time (median PFS: 12 months Cohort 1vs 18 months Cohort 2 vs 33 months Cohort 3, p<0.0001; median OS: 26 months Cohort 1vs 63 months Cohort 2 vs 83 months Cohort 3, p<0.0001), Figure 1. The improvement in PFS and OS over the 3 time periods was seen in patients receiving early (≤ 12 months from diagnosis) and delayed (>12 months) ASCT, Table 2. OS from diagnosis was no different for early versus delayed transplant in Cohort 1 and Cohort 2 (Cohort 1: median OS 57 months for early vs 56 months for delayed, p=0.24 and Cohort 2: median OS 81 months for early vs 82 months for delayed, p=0.70) and showed a trend towards a marginal benefit for delayed transplant in Cohort 3 (median OS 105 months for early vs 109 months for delayed, p=0.046).Treatment related mortality (100 days post transplant) improved over time (7.6% Cohort 1 vs 1.8% Cohort 2 vs 0.7% Cohort 3, p<0.0001), Figure 2A. Mortality within 12 months of ASCT also improved over time (24% Cohort 1vs 8% Cohort 2 vs 6% Cohort 3, p<0.0001). A landmark analysis of OS from 12 months post ASCT continued to reveal improved survival over time(median OS 26 months Cohort 1vs 63 months Cohort 2 vs 83 month Cohort 3, p<0.0001), Figure 2B.
Conclusion: ASCT remains an important therapeutic option for eligible patients with multiple myeloma. There has been a marked improvement over time in PFS and OS and reduction in treatment related mortality and early mortality in myeloma patients receiving ASCT. This is likely to reflect the addition of novel agents to induction regimens and improved post transplant therapies. Despite these improvements myeloma remains incurable with patients continuing to relapse and die many years after ASCT.
Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Lacy:Celgene: Research Funding. Dingli:Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Millennium Takeda: Research Funding; Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Kumar:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding. Gertz:Research to Practice: Consultancy; Physicians Education Resource: Consultancy; Amgen: Consultancy; spectrum: Consultancy, Honoraria; celgene: Consultancy; annexon: Consultancy; janssen: Consultancy; Alnylam: Honoraria; Abbvie: Consultancy; Ionis: Honoraria; Teva: Consultancy; Apellis: Consultancy; Medscape: Consultancy; Prothena: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal